COVID-19: CON-VINCE study enters homestretch
12 April 2021
Originally launched in April 2020 under the aegis of the Research Luxembourg COVID-19 Taskforce, the CON-VINCE study aims to evaluate the prevalence and dynamics of the spread of COVID-19 within the Luxembourgish population, with a specific focus on asymptomatic and mildly symptomatic individuals. The last round of testing of the CON-VINCE participants is due to start in April 2021, approximately one year after the first set of visits upon inclusion in the study. The final wave will provide a comprehensive insight into the evolution and transmission of the disease over an extended timeframe, particularly from an immunity perspective.
Under the leadership of Prof Rejko Krüger, Director of Transversal Translational Medicine (TTM) at the Luxembourg Institute of Health (LIH), CON-VINCE aims to detect asymptomatic and mildly symptomatic (oligosymptomatic) carriers by testing a panel of over 1,800 individuals, representative of the Luxembourgish population, for the presence of the SARS-CoV-2 virus and monitoring them over 12 months through a series of follow-up visits.
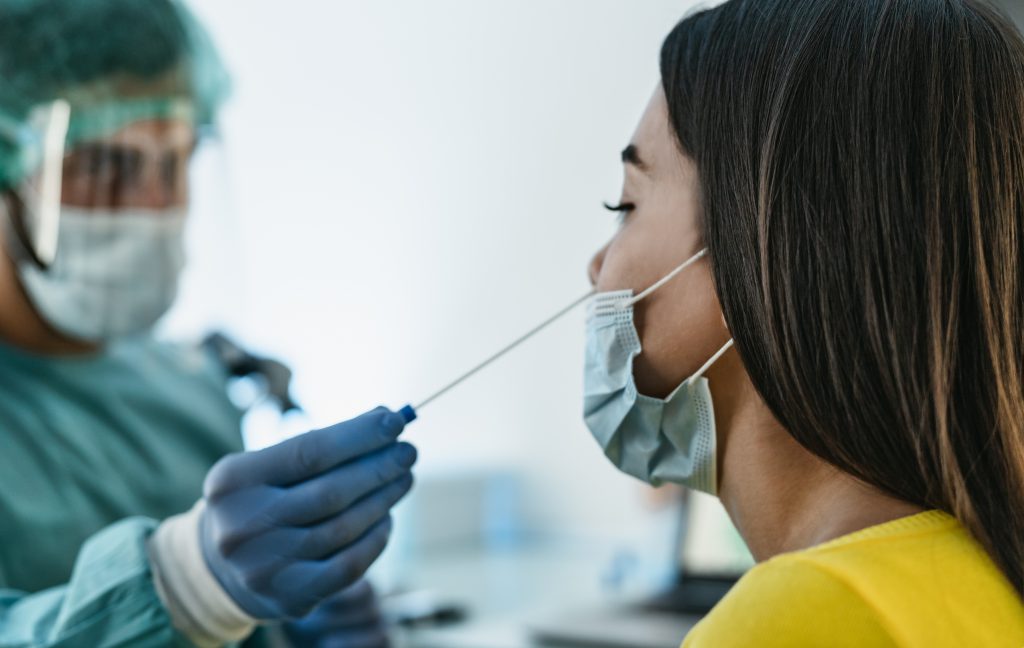
The annual follow-up testing phase under the project will begin on April 19th and is set to run over 5 to 6 weeks. As with the previous rounds of testing, all participants will be subjected once to a nasopharyngeal swab. Blood and stool samples will also be collected once as part of these follow-up visits, with the support of the laboratories Ketterthill, Laboratoires Réunis, BioneXt Lab, as well as of LIH and Laboratoire National de Santé (LNS) as associated partners for biospecimen collection. Biological sampling will be complemented by collecting additional information on confinement measures and vaccination through short follow-up questionnaires.
“From an operational perspective, participants will be asked to fill out the questionnaire provided through our partner TNS-Ilres. Upon completion, they will receive a voucher for sample collection at one of our partner laboratories. Collected samples will then be sent to the Integrated Biobank of Luxembourg (IBBL) for further analysis and storage”, explains Prof Rejko Krüger, coordinator of CON-VINCE.
Specifically, the collected nasopharyngeal swabs will undergo PCR testing to detect the presence of the SARS-CoV-2 virus, while blood samples will be analysed for antibodies (serological testing) to assess whether the participants have mounted an immune response following exposure to the virus or after vaccination.
“We are expecting to obtain crucial information from this annual follow-up, particularly as pertains to the persistence of the antibody response over a full year. Moreover, this last visit will also allow us to analyse cell-based immunity, thereby giving us a more complete picture of the global immune response against the novel SARS-CoV-2”, adds Prof Krüger.
“For this reason, we would like to express our heartfelt gratitude to our partners, and specifically to the diagnostic laboratories and TNS-Ilres, for their unfaltering support and seamless collaboration throughout the past year, as well as to all volunteers who agreed to participate in the study. I take this opportunity to stress again the importance of their renewed participation, particularly in the context of this final wave, without which we would not be able to generate meaningful data and research outcomes for patients and the population in general”, he concludes.
About CON-VINCE
CON-VINCE was launched in April 2020 as one of the several initiatives put in place under the aegis of the Research Luxembourg COVID-19 Taskforce to help contain the current pandemic. By screening a statistically representative panel of volunteers for the presence of the SARS-CoV-2 virus, CON-VINCE will identify asymptomatic and mildly symptomatic individuals and follow them up for a year. Ultimately, the study aims to generate accurate data on the prevalence and transmission of the disease within the Luxembourgish population.
CON-VINCE is led by a consortium of Luxembourgish research institutions, including LIH, its Integrated Biobank of Luxembourg (IBBL), the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg and the Laboratoire National de Santé (LNS), with the support of the market research company TNS-ILRES for the selection of participants and of the national diagnostic laboratories Ketterthill, Laboratoires Réunis and BioneXt Lab as associated partners for sample collection. The study is co-funded by the Luxembourg National Research Fund (FNR) with an amount of EUR 1.4 million and by the Fondation André Losch through a financial commitment of EUR 800,000.
Learn more on the CON-VINCE study